Новини
Головна >>> Публікації
Діагностика інфікованого перелому: чинні концепції та рекомендації
Джерело: Переклад українською та адаптація АО Травма Україна
http://europepmc.org/article/MED/31855973
Geertje A. M. Govaert, MD, PhD (к.м.н., док.мед. Гертьє А.М. Говаерт), Richard Kuehl, MD (док.мед. Річард Куель), Bridget L. Atkins, MD (док.мед. Бріджет Л.Аткінс), Andrej Trampuz, MD (док.мед. Андрей Трампуш), Mario Morgenstern, MD (док.мед. Маріо Моргенштерн), William T. Obremskey, MD, MPH (док.мед., магістр охорони здоров’я Вільям Т.Обремскей), Michael H. J. Verhofstad, MD, PhD (к.м.н., док.мед. Мішель Х.Дж. Ферхофштад), Martin A. McNally, MD, FRCS(Orth) (док.мед, член Королівської колегії хірургів (орт.) Мартін А. Мак-Неллі), та Willem-Jan Metsemakers, MD, PhD (к.м.н., док.мед. Віллем-Жан Мецемейкерз) від робочої групи інфікованих переломів
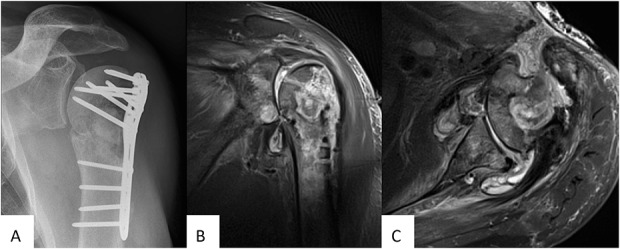
Резюме.
Інфікування перелому є тяжким ускладненням після травми кістки та може становити серйозні проблеми для діагностики. Загалом, існує досить обмежена кількість науково обґрунтованих діагностичних критеріїв інфекції перелому. З огляду на це, «AO Foundation», спільно з Асоціацією суглобових та кісткових інфекцій запропонувала спільне визначення інфекцій переломів, щоб стандартизувати діагностичні критерії та підвищити якість лікування пацієнта і практичне застосування майбутніх досліджень цього стану. Мета даної статті – підсумувати усі доступні діагностичні ознаки та надати рекомендації щодо діагностування інфекції переломів. Для цього співавтори в ході дискусій прийшли до спільного визначення та запропонували удосконалення на підставі доступних доказів діагностичної цінності клінічних параметрів, маркерів запалення в плазмі крові, методів візуалізації, взяття зразків тканин та рідин після обробки ультразвуком, молекулярних біотехнологій та гістопатологічних досліджень. Крім того, буде надано рекомендації щодо взяття мікробіологічних зразків та оперативних лабораторних процедур у випадках інфікування переломів.
Ключові слова: інфікований перелом, діагноз, діагностичні критерії, визначення, клінічні критерії, медична візуалізація, гістопатологія, мікробіологія, маркери запалення в плазмі крові, перелом, інфекція.
Рівень доказовості: діагностичний рівень V. Повний опис рівнів доказовості наведено в інструкціях для авторів.
Докладніше з матеріалом можна ознайомитися за посиланням http://europepmc.org/article/MED/31855973 (Free full text)
REFERENCES
2. Metsemakers WJ, Moriarty TF, Morgenstern M, et al. Letter to the editor: new definition for periprosthetic joint infection: from the workgroup of the musculoskeletal infection Society. Clin Orthop Relat Res. 2016;474:2726–2727. [Europe PMC free article] [Abstract] [Google Scholar]
3. Parvizi J, Zmistowski B, Berbari EF, et al. New definition for periprosthetic joint infection: from the Workgroup of the Musculoskeletal Infection Society. Clin Orthop Relat Res. 2011;469:2992–2994. [Europe PMC free article] [Abstract] [Google Scholar]
4. Parvizi J, Tan TL, Goswami K, et al. The 2018 definition of periprosthetic hip and knee infection: an evidence-based and validated criteria. J Arthroplasty. 2018;33:1309–1314 e1302. [Abstract] [Google Scholar]
5. Morgenstern M, Moriarty TF, Kuehl R, et al. International survey among orthopaedic trauma surgeons: lack of a definition of fracture-related infection. Injury. 2018;49:491–496. [Abstract] [Google Scholar]
6. Govaert GAM, Glaudemans A, Ploegmakers JJW, et al. Diagnostic strategies for posttraumatic osteomyelitis: a survey amongst Dutch medical specialists demonstrates the need for a consensus protocol. Eur J Trauma Emerg Surg. 2018;44:417–426. [Europe PMC free article] [Abstract] [Google Scholar]
7. Metsemakers WJ, Morgenstern M, McNally MA, et al. Fracture-related infection: a consensus on definition from an international expert group. Injury. 2018;49:505–510. [Abstract] [Google Scholar]
8. Cats-Baril W, Gehrke T, Huff K, et al. International consensus on periprosthetic joint infection: description of the consensus process. Clin Orthop Relat Res. 2013;471:4065–4075. [Europe PMC free article] [Abstract] [Google Scholar]
9. Bezstarosti H, Van Lieshout EMM, Voskamp LW, et al. Insights into treatment and outcome of fracture-related infection: a systematic literature review. Arch Orthop Trauma Surg. 2019;139:61–72. [Europe PMC free article] [Abstract] [Google Scholar]
10. Nicolas-Avila JA, Adrover JM, Hidalgo A. Neutrophils in homeostasis, immunity, and cancer. Immunity. 2017;46:15–28. [Abstract] [Google Scholar]
11. Teng TS, Ji AL, Ji XY, et al. Neutrophils and immunity: from bactericidal action to being conquered. J Immunol Res. 2017;2017:9671604. [Europe PMC free article] [Abstract] [Google Scholar]
12. Kraft CN, Kruger T, Westhoff J, et al. CRP and leukocyte-count after lumbar spine surgery: fusion vs. nucleotomy. Acta Orthop. 2011;82:489–493. [Europe PMC free article] [Abstract] [Google Scholar]
13. Sproston NR, Ashworth JJ. Role of C-reactive protein at sites of inflammation and infection. Front Immunol. 2018;9:754. [Europe PMC free article] [Abstract] [Google Scholar]
14. Lu J, Marnell LL, Marjon KD, et al. Structural recognition and functional activation of FcgammaR by innate pentraxins. Nature. 2008;456:989–992. [Europe PMC free article] [Abstract] [Google Scholar]
15. Neumaier M, Scherer MA. C-reactive protein levels for early detection of postoperative infection after fracture surgery in 787 patients. Acta Orthop. 2008;79:428–432. [Abstract] [Google Scholar]
16. Kunakornsawat S, Tungsiripat R, Putthiwara D, et al. Postoperative kinetics of C-reactive protein and erythrocyte sediment rate in one-, two-, and multilevel posterior spinal decompressions and instrumentations. Global Spine J. 2017;7:448–451. [Europe PMC free article] [Abstract] [Google Scholar]
17. Brown KA, Brain SD, Pearson JD, et al. Neutrophils in development of multiple organ failure in sepsis. Lancet. 2006;368:157–169. [Abstract] [Google Scholar]
18. Chmielewski PP, Strzelec B. Elevated leukocyte count as a harbinger of systemic inflammation, disease progression, and poor prognosis: a review. Folia Morphol (Warsz). 2018;77:171–178. [Abstract] [Google Scholar]
19. Ettinger M, Calliess T, Kielstein JT, et al. Circulating biomarkers for discrimination between aseptic joint failure, low-grade infection, and high-grade septic failure. Clin Infect Dis. 2015;61:332–341. [Abstract] [Google Scholar]
20. Fischer CL, Gill C, Forrester MG, et al. Quantitation of «acute-phase proteins» postoperatively: value in detection and monitoring of complications. Am J Clin Pathol. 1976;66:840–846. [Abstract] [Google Scholar]
21. Scherer MA, Neumaier M, von Gumppenberg S. C-reactive protein in patients who had operative fracture treatment. Clin Orthop Relat Res. 2001:287–293. [Abstract] [Google Scholar]
22. van den Kieboom J, Bosch P, Plate JDJ, et al. Diagnostic accuracy of serum inflammatory markers in late fracture-related infection: a systematic review and meta-analysis. Bone Joint J. 2018;100B:1542–1550. [Abstract] [Google Scholar]
23. Govaert GA, FF IJ, McNally M, et al. Accuracy of diagnostic imaging modalities for peripheral post-traumatic osteomyelitis—a systematic review of the recent literature. Eur J Nucl Med Mol Imaging. 2017;44:1393–1407. [Europe PMC free article] [Abstract] [Google Scholar]
24. Goebel M, Rosa F, Tatsch K, et al. Diagnosis of chronic osteitis of the bones in the extremities: relative value of F-18 FDG-PET [in German]. Unfallchirurg. 2007;110:859–866. [Abstract] [Google Scholar]
25. Kaim A, Ledermann HP, Bongartz G, et al. Chronic post-traumatic osteomyelitis of the lower extremity: comparison of magnetic resonance imaging and combined bone scintigraphy/immunoscintigraphy with radiolabelled monoclonal antigranulocyte antibodies. Skeletal Radiol. 2000;29:378–386. [Abstract] [Google Scholar]
26. Govaert GA, Glaudemans AW. Nuclear medicine imaging of posttraumatic osteomyelitis. Eur J Trauma Emerg Surg. 2016;42:397–410. [Europe PMC free article] [Abstract] [Google Scholar]
27. Glaudemans AW, Prandini N, DI Girolamo M, et al. Hybrid imaging of musculoskeletal infections. Q J Nucl Med Mol Imaging. 2018;62:3–13. [Abstract] [Google Scholar]
28. Ballani NS, Al-Huda FA, Khan HA, et al. The value of quantitative uptake of (99m)Tc-MDP and (99m)Tc-HMPAO white blood cells in detecting osteomyelitis in violated peripheral bones. J Nucl Med Technol. 2007;35:91–95. [Abstract] [Google Scholar]
29. Meller J, Koster G, Liersch T, et al. Chronic bacterial osteomyelitis: prospective comparison of (18)F-FDG imaging with a dual-head coincidence camera and (111)In-labelled autologous leucocyte scintigraphy. Eur J Nucl Med Mol Imaging. 2002;29:53–60. [Abstract] [Google Scholar]
30. Glaudemans AW, de Vries EF, Vermeulen LE, et al. A large retrospective single-centre study to define the best image acquisition protocols and interpretation criteria for white blood cell scintigraphy with (9)(9)mTc-HMPAO-labelled leucocytes in musculoskeletal infections. Eur J Nucl Med Mol Imaging. 2013;40:1760–1769. [Abstract] [Google Scholar]
31. Horger M, Eschmann SM, Pfannenberg C, et al. The value of SPET/CT in chronic osteomyelitis. Eur J Nucl Med Mol Imaging. 2003;30:1665–1673. [Abstract] [Google Scholar]
32. Govaert GAM, Bosch P, FFA IJ, et al. High diagnostic accuracy of white blood cell scintigraphy for fracture related infections: results of a large retrospective single-center study. Injury. 2018;49:1085–1090. [Abstract] [Google Scholar]
33. Glaudemans AW, Galli F, Pacilio M, et al. Leukocyte and bacteria imaging in prosthetic joint infection. Eur Cell Mater. 2013;25:61–77. [Abstract] [Google Scholar]
34. Lemans JVC, Hobbelink MGG, FFA IJ, et al. The diagnostic accuracy of (18)F-FDG PET/CT in diagnosing fracture-related infections. Eur J Nucl Med Mol Imaging. 2019;46:999–1008. [Europe PMC free article] [Abstract] [Google Scholar]
35. Hartmann A, Eid K, Dora C, et al. Diagnostic value of 18F-FDG PET/CT in trauma patients with suspected chronic osteomyelitis. Eur J Nucl Med Mol Imaging. 2007;34:704–714. [Abstract] [Google Scholar]
36. Shemesh S, Kosashvili Y, Groshar D, et al. The value of 18-FDG PET/CT in the diagnosis and management of implant-related infections of the tibia: a case series. Injury. 2015;46:1377–1382. [Abstract] [Google Scholar]
37. van Vliet KE, de Jong VM, Termaat MF, et al. FDG-PET/CT for differentiating between aseptic and septic delayed union in the lower extremity. Arch Orthop Trauma Surg. 2018;138:189–194. [Europe PMC free article] [Abstract] [Google Scholar]
38. Wenter V, Muller JP, Albert NL, et al. The diagnostic value of [(18)F]FDG PET for the detection of chronic osteomyelitis and implant-associated infection. Eur J Nucl Med Mol Imaging. 2016;43:749–761. [Abstract] [Google Scholar]
39. Atkins BL, Athanasou N, Deeks JJ, et al. Prospective evaluation of criteria for microbiological diagnosis of prosthetic-joint infection at revision arthroplasty: the OSIRIS Collaborative Study Group. J Clin Microbiol. 1998;36:2932–2939. [Europe PMC free article] [Abstract] [Google Scholar]
40. McNally MA. Infection after fracture. In: Kates SL, Borens O, eds Principles of Orthopedic Infection Management. New York, NY: AO Trauma Thieme Verlag; 2016:139–165. [Google Scholar]
41. Hellebrekers P, Rentenaar RJ, McNally MA, et al. Getting it right first time: the importance of a structured tissue sampling protocol for diagnosing fracture-related infections. Injury. 2019. [epub ahead of print]. [Abstract] [Google Scholar]
42. Perez-Prieto D, Portillo ME, Puig-Verdie L, et al. Preoperative antibiotic prophylaxis in prosthetic joint infections: not a concern for intraoperative cultures. Diagn Microbiol Infect Dis. 2016;86:442–445. [Abstract] [Google Scholar]
43. Bedencic K, Kavcic M, Faganeli N, et al. Does preoperative antimicrobial prophylaxis influence the diagnostic potential of periprosthetic tissues in hip or knee infections? Clin Orthop Relat Res. 2016;474:258–264. [Europe PMC free article] [Abstract] [Google Scholar]
44. Wouthuyzen-Bakker M, Tornero E, Claret G, et al. Withholding preoperative antibiotic prophylaxis in knee prosthesis revision: a retrospective analysis on culture results and risk of infection. J Arthroplasty. 2017;32:2829–2833. [Abstract] [Google Scholar]
45. Miller NS, Rogan D, Orr BL, et al. Comparison of BD Bactec Plus blood culture media to VersaTREK Redox blood culture media for detection of bacterial pathogens in simulated adult blood cultures containing therapeutic concentrations of antibiotics. J Clin Microbiol. 2011;49:1624–1627. [Europe PMC free article] [Abstract] [Google Scholar]
46. Grupper M, Nicolau DP, Aslanzadeh J, et al. Effects of clinically meaningful concentrations of antipseudomonal beta-lactams on time to detection and organism growth in blood culture bottles. J Clin Microbiol. 2017;55:3502–3512. [Europe PMC free article] [Abstract] [Google Scholar]
47. Dudareva M, Barrett L, Figtree M, et al. Sonication versus tissue sampling for diagnosis of prosthetic joint and other orthopaedic device-related infections. J Clin Microbiol. 2018;56. [Europe PMC free article] [Abstract] [Google Scholar]
48. Dudareva M, Barrett L, Morgenstern M, et al. An evidence base for tissue sampling and culture interpretation in fracture-related infection. Orthop Proc. 2018;100B:15. [Google Scholar]
49. Mackowiak PA, Jones SR, Smith JW. Diagnostic value of sinus-tract cultures in chronic osteomyelitis. JAMA. 1978;239:2772–2775. [Abstract] [Google Scholar]
50. Aggarwal VK, Higuera C, Deirmengian G, et al. Swab cultures are not as effective as tissue cultures for diagnosis of periprosthetic joint infection. Clin Orthop Relat Res. 2013;471:3196–3203. [Europe PMC free article] [Abstract] [Google Scholar]
51. Schlung JE, Bastrom TP, Roocroft JH, et al. Femoral neck aspiration aids in the diagnosis of osteomyelitis in children with septic hip. J Pediatr Orthop. 2018;38:532–536. [Abstract] [Google Scholar]
52. Pupaibool J, Vasoo S, Erwin PJ, et al. The utility of image-guided percutaneous needle aspiration biopsy for the diagnosis of spontaneous vertebral osteomyelitis: a systematic review and meta-analysis. Spine J. 2015;15:122–131. [Abstract] [Google Scholar]
53. Beroukhim G, Shah R, Bucknor MD. Factors predicting positive culture in CT-guided bone biopsy performed for suspected osteomyelitis. AJR Am J Roentgenol. 2019;212:620–624. [Abstract] [Google Scholar]
54. Hoang D, Fisher S, Oz OK, et al. Percutaneous CT guided bone biopsy for suspected osteomyelitis: diagnostic yield and impact on patient’s treatment change and recovery. Eur J Radiol. 2019;114:85–91. [Abstract] [Google Scholar]
55. Said N, Chalian M, Fox MG, et al. Percutaneous image-guided bone biopsy of osteomyelitis in the foot and pelvis has a low impact on guiding antibiotics management: a retrospective analysis of 60 bone biopsies. Skeletal Radiol. 2019;48:1385–1391. [Abstract] [Google Scholar]
56. Wu JS, Gorbachova T, Morrison WB, et al. Imaging-guided bone biopsy for osteomyelitis: are there factors associated with positive or negative cultures? AJR Am J Roentgenol. 2007;188:1529–1534. [Abstract] [Google Scholar]
57. Peel TN, Dylla BL, Hughes JG, et al. Improved diagnosis of prosthetic joint infection by culturing periprosthetic tissue specimens in blood culture bottles. MBio. 2016;7:e01776–e01715. [Europe PMC free article] [Abstract] [Google Scholar]
58. Roux AL, Sivadon-Tardy V, Bauer T, et al. Diagnosis of prosthetic joint infection by beadmill processing of a periprosthetic specimen. Clin Microbiol Infect. 2011;17:447–450. [Abstract] [Google Scholar]
59. Suren C, Harrasser N, Pohlig F, et al. Prospective analysis of a sterile, semi-automated tissue biopsy homogenization method in the diagnosis of prosthetic joint infections. In Vivo. 2017;31:937–942. [Europe PMC free article] [Abstract] [Google Scholar]
60. Hughes HC, Newnham R, Athanasou N, et al. Microbiological diagnosis of prosthetic joint infections: a prospective evaluation of four bacterial culture media in the routine laboratory. Clin Microbiol Infect. 2011;17:1528–1530. [Abstract] [Google Scholar]
61. Schafer P, Fink B, Sandow D, et al. Prolonged bacterial culture to identify late periprosthetic joint infection: a promising strategy. Clin Infect Dis. 2008;47:1403–1409. [Abstract] [Google Scholar]
62. Minassian AM, Newnham R, Kalimeris E, et al. Use of an automated blood culture system (BD BACTEC) for diagnosis of prosthetic joint infections: easy and fast. BMC Infect Dis. 2014;14:233. [Europe PMC free article] [Abstract] [Google Scholar]
63. Patel R. MALDI-TOF MS for the diagnosis of infectious diseases. Clin Chem. 2015;61:100–111. [Abstract] [Google Scholar]
64. Trampuz A, Piper KE, Jacobson MJ, et al. Sonication of removed hip and knee prostheses for diagnosis of infection. N Engl J Med. 2007;357:654–663. [Abstract] [Google Scholar]
65. Jonsson EO, Johannesdottir H, Robertsson O, et al. Bacterial contamination of the wound during primary total hip and knee replacement: median 13 years of follow-up of 90 replacements. Acta Orthop. 2014;85:159–164. [Europe PMC free article] [Abstract] [Google Scholar]
66. Onsea J, Depypere M, Govaert G, et al. Accuracy of tissue and sonication fluid sampling for the diagnosis of fracture-related infection: a systematic review and critical appraisal. J Bone Joint Infect. 2018;3:173–181. [Europe PMC free article] [Abstract] [Google Scholar]
67. Hartley JC, Harris KA. Molecular techniques for diagnosing prosthetic joint infections. J Antimicrob Chemother. 2014;69(suppl 1):i21–i24. [Abstract] [Google Scholar]
68. Morgenstern C, Cabric S, Perka C, et al. Synovial fluid multiplex PCR is superior to culture for detection of low-virulent pathogens causing periprosthetic joint infection. Diagn Microbiol Infect Dis. 2018;90:115–119. [Abstract] [Google Scholar]
69. Morgenstern M, Kuhl R, Eckardt H, et al. Diagnostic challenges and future perspectives in fracture-related infection. Injury. 2018;49(suppl 1):S83–S90. [Abstract] [Google Scholar]
70. Omar M, Suero EM, Liodakis E, et al. Diagnostic performance of swab PCR as an alternative to tissue culture methods for diagnosing infections associated with fracture fixation devices. Injury. 2016;47:1421–1426. [Abstract] [Google Scholar]
71. Renz N, Cabric S, Morgenstern C, et al. Value of PCR in sonication fluid for the diagnosis of orthopedic hardware-associated infections: has the molecular era arrived? Injury. 2018;49:806–811. [Abstract] [Google Scholar]
72. Morgenstern M, Athanasou NA, Ferguson JY, et al. The value of quantitative histology in the diagnosis of fracture-related infection. Bone Joint J. 2018;100B:966–972. [Abstract] [Google Scholar]
73. Govaert GAM, Hobbelink MGG, Reininga IHF, et al. The accuracy of diagnostic imaging techniques in patients with a suspected fracture-related infection (IFI) trial: study protocol for a prospective multicenter cohort study. BMJ Open. 2019;9:e027772. [Europe PMC free article] [Abstract]
* Переклад матеріалу з англійської зроблено ексклюзивно для проекту «Травматологія та фармакологія: точки дотику».
Залишайтеся на зв'язку !
Зв’яжіться з нами і станьте учасником AO Trauma Україна або підпишіться, щоб отримувати останні новини та оновлення



